Be Healthy and Live Life to its Fullest
By Rick Mauch, Sports Journalist
Mandy Marquardt is racing for her life – and the lives of many others.
Mandy lives with Type 1 Diabetes. Yes, it presents a daily challenge, but like all championship-caliber athletes and their competition, she has found a way to control it instead of it being in control of her.
Read the Full Article: Click Here
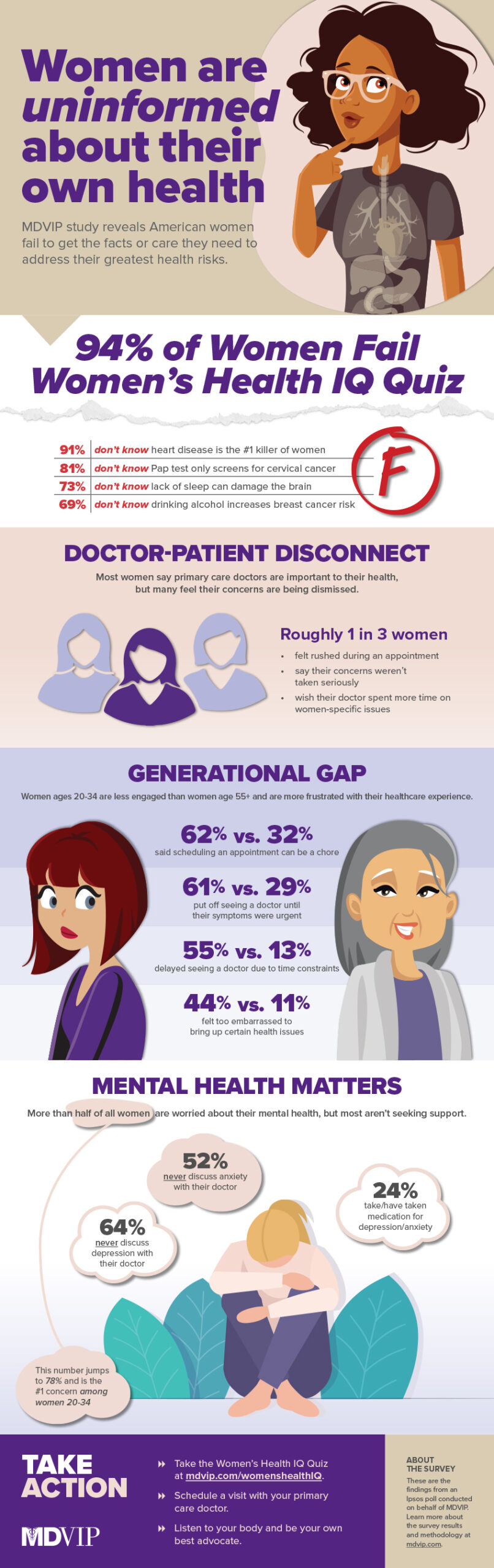
New Study Finds Alarming Gaps in Women's Health Knowledge and Healthcare Experiences
MDVIP/Ipsos Survey Finds 9 in 10 Women Fail ‘Women’s Health IQ’ Quiz
COVID-19 Pandemic Challenging Women’s Mental Health, But Also Strengthening Their Resilience
Although women make the lion’s share of healthcare decisions in the U.S., most are in the dark when it comes to their own health, according to a new survey on women’s health from MDVIP and Ipsos. A staggering 94 percent of women across all ages 20 and up failed a Women’s Health IQ Quiz, revealing their vast lack of knowledge about the health issues that affect them most, including heart disease, Alzheimer’s and menopause. The study also finds that many women’s concerns are dismissed when seeking medical care, which could explain why more than 2 in 5 women say they have delayed seeing a doctor until their symptoms became urgent (44 percent).
Experience the interactive Multichannel News Release here: https://www.multivu.com/players/English/8727651-mdvip-womens-health-survey/
In addition, the survey shows that the COVID-19 crisis has been especially trying on women’s physical and mental health. Over 2 in 5 admit they’ve developed unhealthy habits (44 percent), and over half say they have felt more stressed, anxious or depressed during the pandemic (53 percent). But there is a silver lining: Nearly 6 in 10 women say they are more resilient as a result of the pandemic, and the same number are motivated to take steps to improve their health (58 percent).
“Women experience disease differently than men, from their symptoms to the way they respond to medication. Yet, our study underscores a pervasive knowledge gap that hinders many women from seeking and receiving the proper care they need,” said Dr. Andrea Klemes, Chief Medical Officer of MDVIP. “One reason is the lack of available data on women, as they’ve historically been underrepresented in clinical research. There has been some progress in narrowing this gap, but our findings reinforce a critical need for more and better data on the role gender plays in health that will enable both providers and patients to address the unique concerns of women across their lifespans.”
Among the findings of the MDVIP Women’s Health Survey emerged three key themes:
1. Women Are Largely Uninformed About Their Health
Most women don’t know the facts about the greatest health risks they face, and many aren’t engaging with their doctors to help identify and mitigate their own risk for disease.
- 9 in 10 don’t know heart disease is the leading cause of death in women (91 percent).
- 8 in 10 don’t know the Pap test only screens for cancer of the cervix (81 percent).
- 7 in 10 don’t know drinking alcohol increases breast cancer risk (69 percent).
- 6 in 10 have never had a mental health screening (59 percent), and most have never discussed depression (64 percent) or stress/anxiety (52 percent) with their doctor.
- 8 in 10 have never been screened for inflammatory markers (78 percent), which can identify risk or presence of heart disease.
2. Many Women, Especially Millennials and Gen Z, Receive Inadequate Treatment
Although 9 in 10 women rank primary care physicians as an important resource in their health (89 percent), many women – especially those ages 20 to 34 – say they aren’t getting adequate time or attention from their doctor.
- 47 percent of women say scheduling an appointment is a chore; jumps to 62 percent among women 20-34.
- 44 percent have put off seeing a doctor until their symptoms were urgent; jumps to 61 percent among women 20-34.
- 31 percent say their concerns weren’t taken seriously; jumps to 45 percent among women 20-34.
- 31 percent have felt rushed and couldn’t ask all their questions; jumps to 47 percent among women 20-34.
- 28 percent say they had a condition that wasn’t properly addressed or diagnosed; jumps to 35 percent among women 20-34.
3. COVID-19 Impacting Women’s Physical and Emotional Health
Nearly 7 in 10 women are concerned about COVID-19 (69 percent) – ahead of cancer (60 percent) and heart disease (55 percent) – while many report the year-long pandemic is wreaking further havoc on their physical and emotional health.
- 53 percent have felt more stressed, anxious or depressed during the pandemic.
- 41 percent say there’ve been times when they felt like they were going to break down.
- 44 percent say they’ve developed unhealthy habits, such as overeating and drinking.
- 28 percent have delayed seeking medical care due to the pandemic.
“Women are the cornerstone of a family’s health, often acting as caregivers to their partners, children and aging parents. But women also need to be proactive and primary advocates for their own health,” added Dr. Klemes. “Women should feel empowered to talk to their doctor about their numbers, their personal risk factors for disease and ways to reduce them. If they feel their concerns are being dismissed, they should speak up or seek a physician who will take the time to listen, provide support and work collaboratively on finding solutions.”
To take the MDVIP Women’s Health IQ Quiz, visit: https://www.mdvip.com/patients/womens-health-center
About the Women’s Health Survey
These are the findings from an Ipsos poll conducted March 5 – 9, 2021, on behalf of MDVIP. For the survey, a sample of 1,466 women ages 20 and over from the continental U.S., Alaska and Hawaii were interviewed online in English. The precision of Ipsos online polls is measured using a credibility interval. In this case, the poll has a credibility interval of plus or minus 2.9 percentage points for all respondents. For more information about Ipsos, please visit https://www.ipsos.com/en-us.
About MDVIP
MDVIP leads the market in membership-based healthcare that goes far beyond concierge medicine services with a national network of over 1,100 primary care physicians serving more than 350,000 patients. MDVIP-affiliated physicians limit the size of their practices, which affords them the time needed to provide patients with more individualized service and attention, including an annual, comprehensive preventive care program and customized wellness plan. Published research shows that the MDVIP primary care model identifies more patients at risk for cardiovascular disease, delivers more preventive health services and saves the healthcare system millions of dollars through reduced hospitalizations and readmissions. In response to the growing consumer demand for a more personalized healthcare experience, hospital systems are incorporating the MDVIP model into their primary care offering. The organization, which celebrated its 20th anniversary last year, has been a Great Place to Work-Certified® company since 2018 and is recognized by Fortune as one of the 2021 Best Workplaces in Healthcare. For more information, visit www.mdvip.com. Follow MDVIP on Facebook, Twitter and LinkedIn.
INVO Bioscience Appoints Meryle Lynn Chamberlain to Lead Marketing for Global Brand Expansion
INVO Bioscience, Inc. (NASDAQ: INVO), a medical device company focused on commercializing the world’s only in vivo Intravaginal Culture System (IVC), INVOcell®, an effective and affordable treatment for patients diagnosed with infertility, is pleased to announce the appointment of tenured women’s health & fertility solution marketing professional, Meryle Lynn Chamberlain, as Director of Marketing, a newly created position within the company.
Chamberlain brings over 15 years of marketing experience in the women’s health field including her most recent role as a Product Marketing Manager at Wildflower Health, a digital health company focused on women’s health and pregnancy and the Marketing Manager at The Emergency Nurses Association, an international healthcare organization, where she led integrated marketing strategies for all products, services and events. Ms. Chamberlain has a Bachelor of Arts degree in Political Science from Brigham Young University and the Integrated Marketing Certificate from DePaul University.
Chamberlain joined INVO Bioscience to spearhead and expand INVO Bioscience’s global marketing and brand management strategy for INVOcell. “INVOcell is a revolution for patients suffering from infertility,” said Chamberlain. “This is the only fertility treatment that allows fertilization to take place in the woman’s own body, eliminating the need for expensive lab equipment. I’m thrilled that I have the opportunity to help millions of women and couples realize their dreams of parenthood.”
Steve Shum, CEO of INVO Bioscience, commented, “We are excited to have Meryle Lynn, an experienced women’s healthcare marketing professional join the INVO Bioscience team. This is an exciting time in the history of the Company as we look to expand INVOcell’s adoption within the fertility industry through our planned INVO centers and global commercialization partners and provide affordable, expanded care to patients.
“With the recent addition of Rebecca Messina to our board of directors, who comes from an impressive global strategic marketing back-ground as the former Global CMO of Uber, Beam Suntory, and a 20+ year global career with Coca-Cola, along with the appointment of Meryle Lynn to lead our internal efforts, we have substantially strengthened our marketing capabilities to support the INVOcell-only centers and our growing number of distribution partners. I look forward to Meryle Lynn’s contributions to the team.”
About INVO Bioscience
We are a medical device company focused on creating simplified, lower-cost treatments for patients diagnosed with infertility. Our solution, the INVO® Procedure, is a revolutionary in vivo method of vaginal incubation that offers patients a more natural and intimate experience. Our lead product, the INVOcell®, is a patented medical device used in infertility treatment and is considered an Assisted Reproductive Technology (ART). The INVOcell® is the first Intravaginal Culture (IVC) system in the world used for the natural in vivo incubation of eggs and sperm during fertilization and early embryo development, as an alternative to traditional In Vitro Fertilization (IVF) and Intrauterine Insemination (IUI). Our mission is to increase access to care and expand fertility treatment across the globe with a goal to lower the cost of care and increase the availability of care. For more information, please visit http://invobioscience.com/.
Safe Harbor Statement
This release includes forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. The Company invokes the protections of the Private Securities Litigation Reform Act of 1995. All statements regarding our expected future financial position, results of operations, cash flows, financing plans, business strategies, products and services, competitive positions, growth opportunities, plans and objectives of management for future operations, as well as statements that include words such as “anticipate,” “if,” “believe,” “plan,” “estimate,” “expect,” “intend,” “may,” “could,” “should,” “will,” and other similar expressions are forward-looking statements. All forward-looking statements involve risks, uncertainties and contingencies, many of which are beyond our control, which may cause actual results, performance, or achievements to differ materially from anticipated results, performance, or achievements. Factors that may cause actual results to differ materially from those in the forward-looking statements include those set forth in our filings at www.sec.gov. We are under no obligation to (and expressly disclaim any such obligation to) update or alter our forward-looking statements, whether as a result of new information, future events or otherwise.
SOURCE INVO Bioscience, Inc.
Allergan Aesthetics to Acquire Soliton, Expanding Body Contouring Portfolio
Allergan Aesthetics, an AbbVie company (NYSE: ABBV) and Soliton (NASDAQ: SOLY) today announced a definitive agreement under which Allergan Aesthetics will acquire Soliton and RESONICTM, its Rapid Acoustic Pulse device which recently received U.S. Food and Drug Administration (FDA) 510(k) clearance and is a non-invasive treatment for the short-term improvement in the appearance of cellulite. The acquisition of Soliton expands and complements Allergan Aesthetics’ Body Contouring treatment portfolio which includes CoolSculpting® Elite.
The novel platform technology uses non-invasive rapid, high-frequency sound waves to disrupt targeted cellular structures and connective tissue, physically impacting the fibrous septae beneath the skin that contribute to the dimpled appearance of cellulite. In clinical trial data submitted to the FDA, after a single treatment session RESONICTM demonstrated significant improvement and strong patient satisfaction with 92.9 percent of subjects agreeing or strongly agreeing their cellulite appeared improved.
“There is a huge unmet need to address cellulite and effective treatments have been elusive and frustrating for consumers,” said Carrie Strom, President, Global Allergan Aesthetics and Senior Vice President, AbbVie. “Soliton’s technology offers a new, completely non-invasive approach with clinically-proven results to reduce the appearance of cellulite with no patient downtime. The addition of this technology complements Allergan Aesthetics’ portfolio of body contouring treatments. Health care providers will now have another option to address consumers’ aesthetic concerns.”
“Allergan Aesthetics’ brand recognition, global footprint, track record and commitment to developing best-in-class aesthetic treatments makes the Company ideally suited to maximize the commercial potential of the RESONICTM rapid acoustic pulse technology,” said Walter Klemp, Executive Chairman, Soliton. “I am proud of the passion and accomplishments of the Soliton team and thankful for the ongoing support of our investors which have culminated in this transaction. We look forward to working with Allergan Aesthetics to ensure a successful completion of this transaction.”
Under the terms of the transaction, Allergan Aesthetics will pay $22.60 per share in cash for each outstanding share of Soliton. Soliton’s enterprise value for the transaction is approximately $550 million and was approved by the Boards of Directors of both companies. The transaction is subject to customary closing conditions, including clearance by the U.S. antitrust authorities under the Hart-Scott-Rodino Act and approval of Soliton’s shareholders. Guggenheim Securities served as financial advisor to Soliton and Hogan Lovells served as legal counsel to Soliton.
RESONICTM has also received FDA 510(k) clearance for use in conjunction with laser for tattoo removal and has demonstrated clinical results in fibrotic scars.
About Allergan Aesthetics
At Allergan Aesthetics, an AbbVie company, we develop, manufacture, and market a portfolio of leading aesthetics brands and products. Our aesthetics portfolio includes facial injectables, body contouring, plastics, skin care, and more. Our goal is to consistently provide our customers with innovation, education, exceptional service, and a commitment to excellence, all with a personal touch. For more information, visit www.AllerganAesthetics.com.
About AbbVie
AbbVie’s mission is to discover and deliver innovative medicines that solve serious health issues today and address the medical challenges of tomorrow. We strive to have a remarkable impact on people’s lives across several key therapeutic areas: immunology, oncology, neuroscience, eye care, virology, women’s health and gastroenterology, in addition to products and services across its Allergan Aesthetics portfolio. For more information about AbbVie, please visit us at www.abbvie.com. Follow @abbvie on Twitter, Facebook, Instagram, YouTube and LinkedIn.
About Soliton, Inc.
Soliton, Inc. is a medical device company with a novel and proprietary platform technology licensed from The University of Texas on behalf of MD Anderson Cancer Center. Soliton’s first FDA cleared commercial product will use rapid pulses of acoustic shockwaves as an accessory to lasers for the removal of unwanted tattoos and the treatment of cellulite. Soliton is based in Houston, Texas, and is actively engaged in bringing the Rapid Acoustic Pulse (“RAP”) device to the market. The company believes this “Soliton” method has the potential to lower tattoo removal costs for patients, while increasing profitability to practitioners, compared to current laser removal methods. Soliton is investigating potential additional capabilities of the RAP technology. The device is currently cleared in the United States only for use in tattoo removal and cellulite.
For more information about Soliton, please visit: www.soliton.com.
Forward-Looking Statements
Some statements in this news release are, or may be considered, forward-looking statements for purposes of the Private Securities Litigation Reform Act of 1995. The words “believe,” “expect,” “anticipate,” “project” and similar expressions, among others, generally identify forward-looking statements. Each of AbbVie and Soliton cautions that these forward-looking statements are subject to risks and uncertainties that may cause actual results to differ materially from those indicated in the forward-looking statements. Such risks and uncertainties include, but are not limited to, the risk that the proposed transaction may not be completed in a timely manner or at all, which may adversely affect the business and the price of the common stock of each of AbbVie and Soliton, the failure to satisfy any of the conditions to the consummation of the proposed transaction, including the receipt of certain governmental and regulatory approvals, the occurrence of any event, change or other circumstance that could give rise to the termination of the merger agreement, the outcome of any legal proceedings that have been or may be instituted against AbbVie or Soliton related to the proposed transaction, the failure to realize the expected benefits from AbbVie’s acquisition of Soliton, the failure to promptly and effectively integrate Soliton’s business, competition from other products, challenges to intellectual property, difficulties inherent in the research and development process, the difficulty of predicting future clinical results based on prior clinical results, the timing or outcome of FDA approvals or actions, market acceptance of and continued demand for AbbVie’s and Soliton’s products, difficulties or delays in manufacturing, adverse litigation or government action, changes to laws and regulations applicable to our industry and the impact of public health outbreaks, epidemics or pandemics, such as COVID-19. Additional information about the economic, competitive, governmental, technological and other factors that may affect AbbVie’s and Soliton’s operations is set forth in Item 1A, “Risk Factors,” of their respective Annual Reports on Form 10-K, which have been filed with the Securities and Exchange Commission, as updated by each company’s subsequent Quarterly Reports on Form 10-Q. Neither AbbVie nor Soliton undertakes any obligation to release publicly any revisions to forward-looking statements as a result of subsequent events or developments, except as required by law.
Additional Information and Where to Find It
In connection with the proposed transaction, Soliton, Inc. will be filing documents with the SEC, including preliminary and definitive proxy statements relating to the proposed transaction. The definitive proxy statement will be mailed to Soliton stockholders in connection with the proposed transaction. BEFORE MAKING ANY VOTING DECISION, INVESTORS AND SECURITY HOLDERS ARE URGED TO READ THE PRELIMINARY AND DEFINITIVE PROXY STATEMENTS AND ANY OTHER DOCUMENTS TO BE FILED WITH THE SEC IN CONNECTION WITH THE PROPOSED MERGER OR INCORPORTED BY REFERENCE IN THE PROXY STATEMENT WHEN THEY BECOME AVAILABLE BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION ABOUT THE PROPOSED TRANSACTION. Investors and security holders may obtain free copies of these documents (when they are available) and other related documents filed with the SEC at the SEC’s web site at www.sec.gov, and on Soliton’s website at www.soliton.com and clicking on the “Investors” link and then clicking on the “SEC Filings” link. In addition, the proxy statement and other documents may be obtained free of charge by directing a request to Soliton, Inc., Corporate Secretary, 5304 Ashbrook Drive, Houston, Texas 77081, telephone: (844) 705-4866.
Participants in the Solicitation
Soliton and its directors and executive officers may be deemed participants in the solicitation of proxies from the stockholders of Soliton in connection with the proposed transaction. Information regarding Soliton’s directors and executive officers can be found in Soliton’s definitive proxy statement filed with the SEC on March 26, 2021. Additional information regarding the interests of Soliton’s directors and executive officers in the proposed transaction will be included in the proxy statement described above. These documents are available free of charge at the SEC’s web site at www.sec.gov and from Soliton as described above.







